Plastic Microneedle
Technology
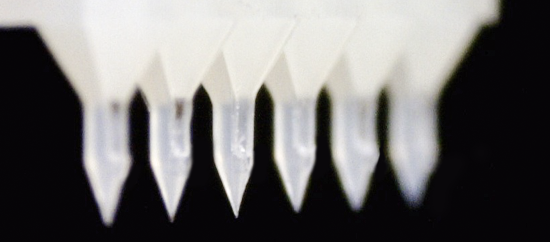
ASTI’s plastic micro needles have high potential for effective vaccination. According to the study with the micro needles, the intradermal vaccination with those has proved the possibility of being more efficacious than conventional subcutaneous injection, because of higher number of antigen-presenting cells in the skin, leading to induction of stronger immune response with lower antigen levels. This means that the amount of vaccine needed can be reduced, therefore saving countless amounts of money, and reducing the waste of precious vaccines. Another aspect of using micro needles for drug injection is that doctors can administrate in a more efficient way, as there are six micro needles, meaning the depth of the injection will be standardized regardless of their skill. In addition, studies show that kind of micro needles could be used to administer erythropoiesis stimulating agent, parathyroid hormone or growth hormone, although vaccination will be the main use of these micro needles.
In short, these micro needles will enable doctors to administrate the drugs intradermally with accuracy and ease.
We are looking for some solid partners in order to further expand our medical business. We would be very keen in finding companies specialized in pharmaceutical products of intradermal administration in order to partner up with.
-

-
Diffusion of blue dye in the upper part of the dermis following injection of 10 μL of 0.1% patent blue solution into porcine skin.
Features
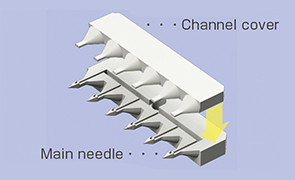
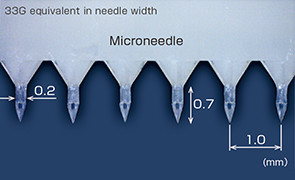
The microneedle has been realized by our mass-producible plastic injection molding, making full use of accumulated micro molding technology, assembly technology, material technology, etc. and has the following features.
-
Fine needle shape and sharp needle tip of 10µm or less
-
Fine hollow flow path realized by stacking procedure (flow path of 80 µm)
-
Highly flexibility in shape of needle
- Transverse or downward flow path opening (hardly to clog)
- Double-ended needle (allows easy drug drawing)
- Reverse shape and arrowhead shape (high tip strength)
-
High hardness biodegradable resin (PGA) * Polyglycolic Acid
- Superior ability of puncture
- Safely handled and easily disposable
-
Making the basic unit (microneedle unit) a row of several needles enables flexible arrangements
- Array
- Oblique insertion
- Lure taper connection
- Medicinal needle connection (double ended)

Study on rats
-
Antibody production following ID and SC administration of mumps vaccine
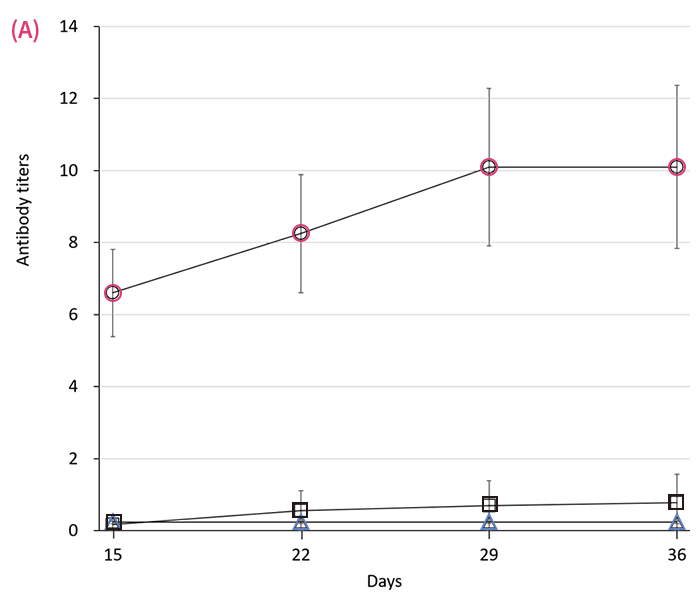
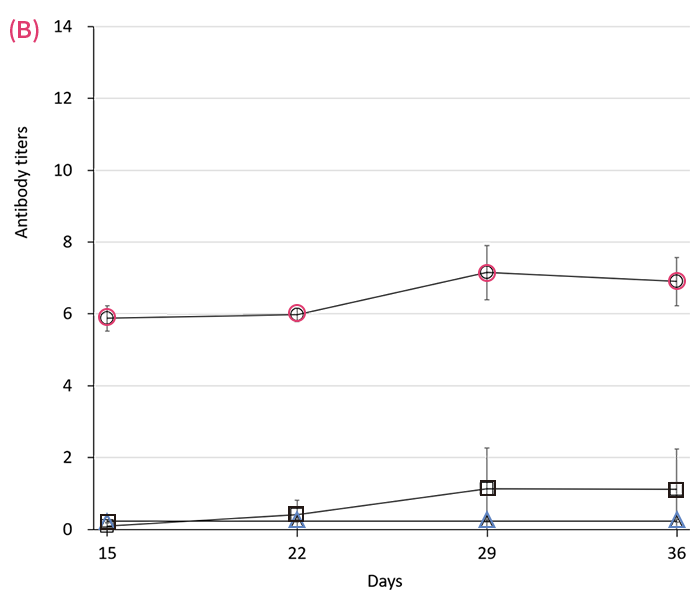
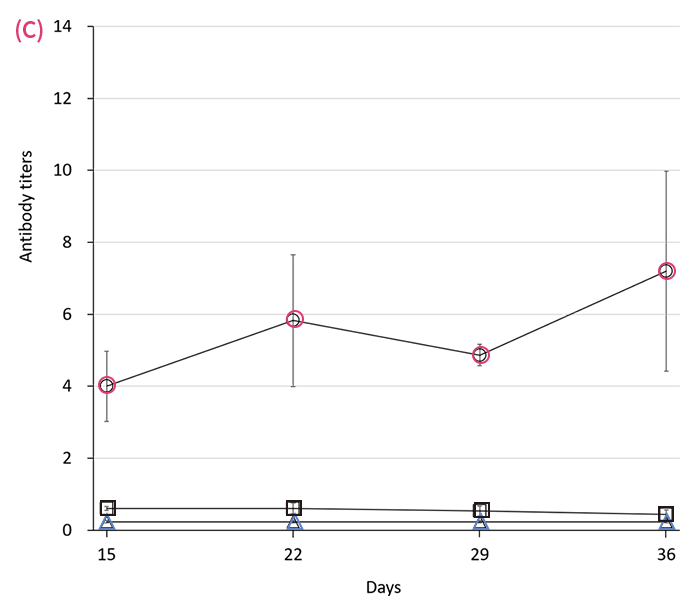
Serum antibody responses in rats following either microneedle () or 27-G needle () immunization with 30 (A), 10 (B) or 3 (C) μL of mumps vaccine in 100 μL of solvent on days 1 and 8. represents the value in control. Antibody titers were measured by EIA every 7 d thereafter. Data are expressed as mean ± SEM
-
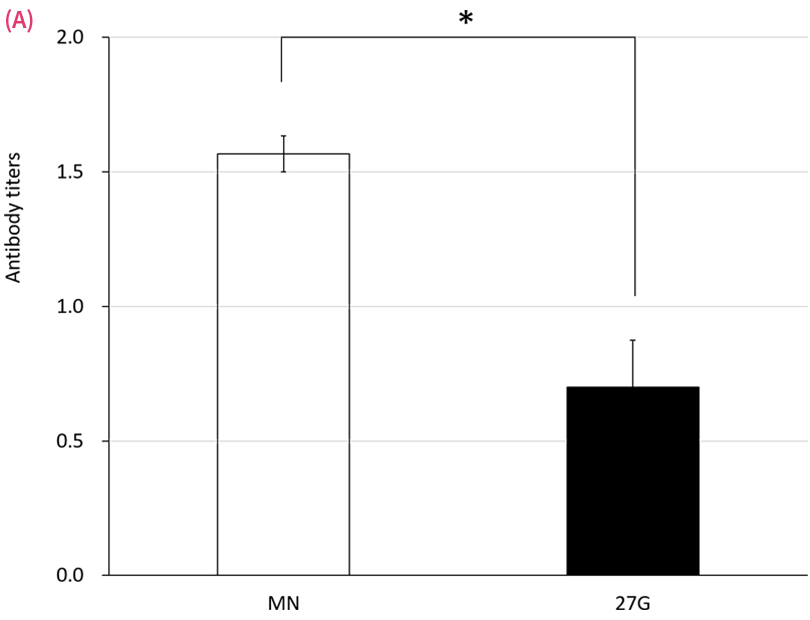
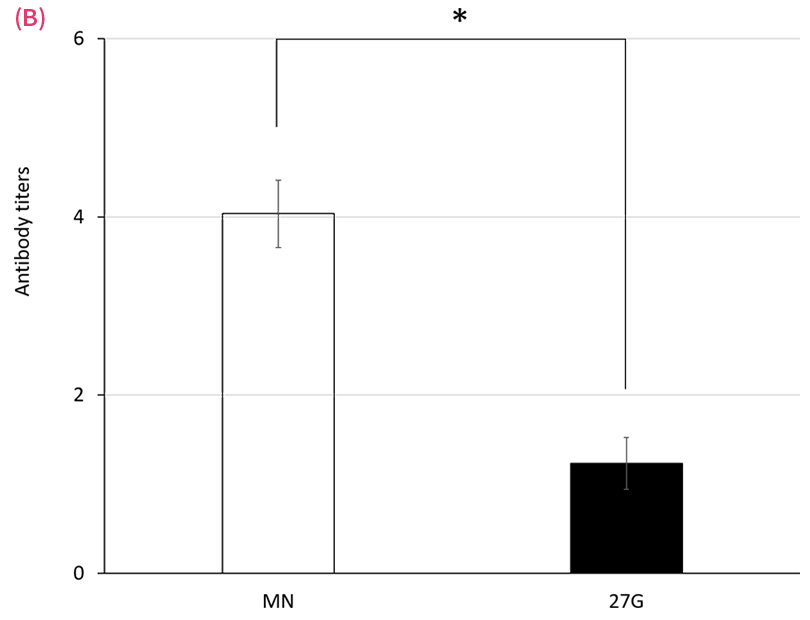
Antibody Production following ID and SC administration of an existing formulation for human use
Serum antibody responses following either microneedle (MN) or 27-G needle (27G) immunization with mumps (A) and varicella (B) vaccines on day 36. Rats were given 20 μL of vaccine in 700 μL of solvent on days 1 and 8. Antibody titers were measured by EIA. Data are expressed as mean ± SEM. *P < .05
Ogai N, Nonaka I, Toda Y, et al. Enhanced immunity in intradermal vaccination by novel hollow microneedles. Skin Res Technol. 2018 Nov;24(4):630-635.